Nipah virus infection outbreak 2018: Treatment and Useful tips for Prevention. Nipah virus (NiV) infection is a newly emerging paramyxoviral zoonosis that causes severe disease in both animals and humans. The natural host of the virus is fruit bats of the Pteropus genus that belong to Pteropodidae Family. Nipah virus out-breaks in the WHO Southeast Asia Region (SEAR Countries): The first outbreak of disease took place in Kampung Sungai Nipah, Malaysia in 1998 and pigs were the inter-mediate hosts. Between September 1998 and June 1999, there was an outbreak of severe viral encephalitis due to Nipah virus in Malaysia. Nipah virus (NiV), a member of the family Paramyxo viridae, and genus Henipavirus was initially isolated and identified in 1999 during an outbreak of encephalitis and respiratory illness among pig farmers and people with close contact with pigs in Malaysia and Singapore.
Consuming date palm sap contaminated by infected fruit bats was the cause of the disease in Bangladesh in 2004 and Human-to-human transmission has also been documented, including in a hospital setting in India. From 1998 to 2015, more than 600 cases of Nipah virus human infections were reported in India and Bangladesh with high fatality.
Earlier Nipah outbreaks in India: Two outbreaks of Nipah virus encephalitis were in the eastern state of West Bengal, in 2001 and 2007. Seventy-one cases with 50 deaths (70% of the cases) were reported in two outbreaks. During the Siliguri outbreak (2001) Nipah virus specific immunoglobulin M (IgM) and IgG antibodies were detected in 9 out of 18 patients. Reverse transcription-polymerase chain reaction (RTPCR) assays detected RNA from NiV in urine samples from 5 patients.
Rapid urbanization and climate changes have caused animals, including bats to lose their natural habitats and they come in contact more frequently with humans, leading to outbreaks of Nipah Virus encephalitis.
A second outbreak was reported in 2007 in Nadia district of West Bengal. Thirty cases of fever with acute respiratory distress and/or neurological symptoms were reported and five cases were fatal and were found to be positive for NiV by RT-PCR. So far, 263 people are infected with 196 deaths since 2001.
Key facts regarding Nipah virus infection:
Nipah virus, RNA virus was first identified as a zoonotic pathogen after an outbreak involving severe respiratory illness in pigs and encephalitic disease in human in Malaysia and Singapore in 1998-1999
Nipah virus is closely related to Hendra virus, both are members of the genus Henipa virus.
Nipah virus causes a range of mild to severe disease in domestic animals such as pigs.
Nipah virus infection in humans causes a range of clinical presentations, from asymptomatic infection (subclinical) to acute respiratory infection and fatal encephalitis.
Nipah virus can be transmitted to humans from animals (bats, pigs), and can also be transmitted directly from humantohuman.
Fruit bats of the Pteropodidae family are the natural host of Nipah virus.
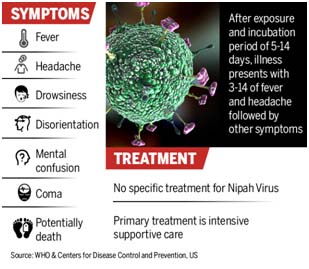
The primary treatment for humans is supportive care. There is no specific treatment or vaccine available for either people or animals.
Nipah virus is on the WHO list of Blueprint priority diseases.
Natural host: fruit bats of the family Pteropodidae – particularly species belonging to the Pteropus genus – are the natural hosts for Nipah virus. There is no apparent disease in fruit bats. Henipavirus infection in Pteropus bats are from Australia, Bangladesh, China Cambodia, India, Indonesia, Madagascar, Malaysia, Papua New Guinea, Thailand and Timor-Leste. African fruit bats of the genus Eidolon, family Pteropodidae, were found positive for antibodies against Nipah and Hendra viruses, indicating that these viruses might be present within the geographic distribution of Pteropodidae bats in Africa.
Nipah virus in domestic animals: Nipah outbreaks in pigs and other domestic animals (horses, goats, sheep, cats and dogs) were first reported during the initial Malaysian outbreak in 1999. Nipah virus is highly contagious in pigs. Pigs are infectious during the incubation period, from 4 to 14 days. An infected pig can exhibit no symptoms, but some develop acute feverish illness, labored breathing, and neurological symptoms such as trembling, twitching and muscle spasms. Generally, mortality was low except in young piglets. These symptoms are not dramatically different from other respiratory and neurological illnesses of pigs. Nipah should be suspected if pigs also have an unusual barking cough or if human cases of encephalitis are present.
Transmission of Nipah virus to humans may occur after direct contact with infected bats, infected pigs, or from other NiV infected people.
Direct transmission:
- Pig-to-human transmission affects pig farmers or abattoir workers by direct human contact with infected andsick pigs, their contaminated tissues and through respiratory droplets, and contact with throat or nasal secretions of sick animals.
- Human-to-human transmission: Health care workers and hospital visitors became ill after exposure to patients hospitalized with NiV illness, suggesting nosocomial infection. Subsequent person-to-person transmission occurred from close physical contact, especially with body fluids, saliva, and respiratory secretions. Urine of infected patients and cerebrospinal fluid (CSF) of encephalitic patients are also source of transmission.
- Bat-to-human transmission: The virus is transmitted either directly or indirectly from infected bats to humans.
Indirect transmission: Drinking of fresh date palm sap, contaminated by saliva, urine and faeces of fruit bats (P.giganteus), is responsible for the transmission of NiV to humans. Pregnancy and In Utero transmission of Nipah Virus play an important role in Vertical Transmission in Henipavirus Epidemiology.
Clinical presentations range from asymptomatic infection to acute respiratory syndrome and fatal encephalitis. After exposure and an incubation period of 5 to 14 days, illness presents with 3-14 days of fever and headache, followed by drowsiness, dis-orientation and mental confusion. These signs and symptoms can progress to coma within 24-48 hours. Initial symptoms of Nipah virus infection mimic those of influenza, causing symptoms like: fever, myalgia, weakness, sore throat, headaches, abdominal pain, nausea, vomiting, swallowing difficulty. Nipah virus may cause serious respiratory symptoms: acute respiratory distress, severe respiratory problems. The neurological symptoms include seizures, dizziness, drowsiness, blurred vision, disorientation, rapid heart rate, severe hypertension, and altered consciousness. Encephalitis can progress into coma and even death. Survivors of brain infection have persistent neurological dysfunction or a relapse of encephalitis with potential complications.
Patients with Nipah virus infection need supportive treatment
Long-term sequelae following Nipah virus infection include persistent convulsions and personality changes. Latent infections with subsequent reactivation of Nipah virus and death have also been reported months and even years after exposure. Psychiatric Complications: Patients develop major depressive disorders following the encephalitis. Cognitive and emotional symptoms of depression, like hopelessness, feelings of worthlessness and suicidal thoughts are common. They also develop personality changes, and may suffer chronic fatigue syndrome. Neuropsychiatric Sequelae of Nipah Virus Encephalitis are:
Psychiatric Evaluation using Schedules for Clinical Assessment in Neuropsychiatry (SCAN)
Neuropsychological Testing: One reviewer assessed attention, visual, and verbal memories during a single session.
Neuroimaging studies using magnetic resonance imaging (MRI) shows unusual multiple small lesions in the cerebral white matter in Nipah virus encephalitis, a pattern unlike other viral encephalitis.
Prevention: 1. Controlling Nipah virus in domestic animals:
Routine and thorough cleaning and disinfection of pig farms (with appropriate detergents) may be effective. In suspected outbreaks, the animal premises and the animals should be quarantined immediately. Restrict or ban the movement of the animals. Culling of infected animals with close supervision of burial or incineration is necessary.
Establish animal health surveillance using a One Health approach to detect new cases and provide an early warning for the veterinary and human public health authorities.
Disinfection of pig farms to reduce the risk of transmission to people.
Restricting or banning the movement of animals from infected farms to other areas will help reduce the spread of the disease.
Reduce the risk of infection in people by raising the awareness of the risk factors and educating people about the measures to reduce exposure and to decrease the infection from NiV.
Public health educational messages should focus on the following:
Reducing the risk of bat to human transmissions:
- Focus on decreasing bat access to date palm sap and to other fresh food products.
- Keeping bats away from sap collection sites with protective coverings (e.g. Bamboo sap skirts)
- Freshly collected date palm juice should be boiled and fruits should be thoroughly washed and peeled before consumption.
Reducing the risk of animal to human transmission:
Gloves and other protective clothing should be worn while handling sick animals or their tissues and during slaughtering or culling procedures.
As much as possible, people should avoid being in contact with infected pigs.
Reducing the risk of human to human transmission:
- Avoid close unprotected physical contact with Nipah virus infected people.
- Regular hand washing should be carried out after caring for or visiting sick people.
The average case fatality rate is 74.5% despite a public awareness campaign and establishment of a referral system for better treatment and nursing care of patients in potential outbreak areas.
- Controlling infection in healthcare settings:
- Healthcare workers caring for patients with suspected or confirmed NiV infection, or handling specimens from them should implement standard infection control precautions at all times.
- Must be handled by trained staff working in suitably equipped laboratories.
- Contact and droplet precautions should be used to avoid nosocomial transmission.
Useful tips for Prevention: Nipah virus infection can be prevented by
- Avoiding exposure to sick pigs and pig handlers in endemic areas
- Fruits and vegetables should be thoroughly washed before eating.
- Fruits bitten by bats or birds should not be eaten.
- Maintain personal hygiene and intensive hand washing practices
- Personal Protective Equipment (PPE) practices should be meticulously followed, preferably use N95 masks while travelling or working in public places to avoid person-to-person transmission.
- Additional efforts focused on surveillance of Acute Encephalitis Syndrome (AES), Influenza like Illness (ILI) and Acute Febrile Illness is very useful in early detection of cases.
- The microbiological cause of AES should be fixed by appropriate laboratory tests.
- Close co-ordination with animal husbandry, forest department and wild life officials.
- Avoid eating raw fruits. Consume well cooked clean home made food till the outbreak settles down
- Awareness will help prevent future outbreaks.
- Surveillance tools should also include reliable laboratory assays for early detection of disease in communities and livestock, and raising awareness of transmission and symptoms is important in reinforcing standard infection control practices to avoid human-to-human infections in hospital settings (nosocomial transmission).
- Precautions should also be taken when submitting and handling laboratory samples, as well as in slaughter houses.
- Seek early health and clinical care for early detection and better care.
Diagnosis: Initial signs and symptoms of NiV infection are non-specific and diagnosis is often not suspected at the time of presentation, a challenge to accurate diagnosis in out break detection. Clinical sample quality, quantity, type, timing of collection and the time necessary to transfer to the laboratory affect the accuracy of the results. NiV infections are diagnosed based on clinical history, examination together with laboratory results during both acute and convalescent phases of the disease. Polymerase chain reaction (RTPCR) from body fluids, antibody detection Via ELISA, Enzyme linked immunosorbent assay (ELISA), polymerase chain reaction (PCR) assay and virus isolation by cell culture are the tests recommended for Nipah virus infection.
Treatment is limited to supportive care. Patients require treatment in an Intensive Care Unit (ICU). Management of respiratory and neurological disease symptoms is the principal course of treatment. Ribavirin, a very broad-spectrum virustatic agent, which showed varying degrees of efficacy against viruses such as respiratory syncytial virus, influenza and measles, was tried on an empirical basis in NiV-infected patients.
A subunit vaccine, using the Hendra G protein, produces cross-protective antibodies against HENV and NIPV has been recently used in Australia to protect horses against Hendra virus. This vaccine offers great potential for henipavirus protection in humans as well. Passive immunization using a human monoclonal antibody targeting the Nipah G glycoprotein has been evaluated in the post-exposure therapy in the ferret model and found to be of benefit.
India (Kerala) Outbreak 2018: Nipah virus is a ‘deadly virus’ that is on ‘top of the list’ of 10 priority diseases identified by WHO as potentials for major outbreak. Kerala state “is in a state of panic after many cases of the killer Nipah virus were detected.” The rare Nipah virus has killed at least 10 people out of 12 confirmed cases in Kerala. Death of a healthcare nurse Lini Puthussery due to the virus has clearly indicated the nosocomial nature of the infection. Experts from the Government of India and World Health Organization have been deployed to the affected areas. Assessment of reports of the teams will help clarify the situation and guide further action. Five point explainer regarding 2018 outbreak:
- The fatality has been reported between 75 per cent and 100 per cent.
- Nipah virus affects the brain. An infected person will have fever, weakness and lethargy.
- Nipah virus infection is an example of a zoonotic disease and If the animal is given adequate antibiotics, the chances of a human being getting the disease will be lesser
- There have been cases of human-to-human transmission too and there is a need to confirm if the transmission happened because everyone was exposed to the same infected person or if the same source passed on the infection to another person.
- The Nipah virus has a tendency to adapt or mutate, like the H1N1 virus. If you get a swine flu or influenza vaccination this year, the effect of the vaccination may not be last through to the next year because the virus would have mutated by then. And that is why such viruses are very deadly. Some of the deadliest diseases in the world are viral-borne diseases.
How can Nipah virus endemic be curbed?
- Being aware and prepared to the Nipah viral endemic can help curbing the viral menace.
- Infective virus easily spreads through bats and their excreta. Avoid eating fruits that are suspected to be infected (dates sap directly from trees harboring the bats).
- Early detection: Raising awareness regarding transmission and symptoms.
- Bats are migratory; the disease may spread to other geographies. Intensive surveillance is thus important.
- Safe infection control practices in hospitals to prevent human-to-human infections.
Emergencies preparedness, response: The roadmap prioritizes the development of counter measures (diagnostics, therapeutics and vaccines) that are most needed by Nipah- affected countries and is the result of extensive consultations with the Nipah R&D roadmap taskforce, leading national and international experts and other key stakeholders. Currently, there’s no specific treatment or a vaccine for Nipah virus infection. Nipah R&D Roadmap has called for comments by Friday, 8 June 2018, with the subject line “Comments on draft Nipah R&D roadmap” (NipahRDRoadmap@who.int).
Conclusion: The circulation of NiV may be influenced by the presence of genetic polymorphisms along the virus genome. This antigenic variability may play an important role in the ability of the virus to escape the host immune response. Monitoring will be important to implement possible intervention strategies. In Asiatic countries, there is a close contact between animals and humans, especially in rural settings. This represents a vulnerability of Asia for outbreaks caused by zoonotic infections. This vulnerability is further increased by socio-cultural beliefs and weak public health infrastructure. The need of a multidisciplinary approach to prevent and control zoonotic infections in these countries is important. Phylogenetic and evolutionary analysis represent promising tools to evidence epidemics, to study their origin and evolution and finally to act with effective preventive measure.

Dr.N.Mariappan